Molecular Shape
A covalent molecule may have any of the following shapes. (The central atom in a molecule is typically the most electronegative.)
Linear
The central atom has two atoms bonded to it and no lone pairs.
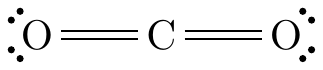
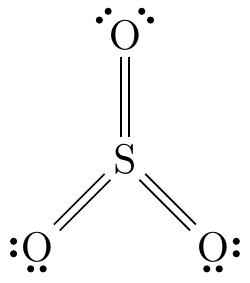
Trigonal planar
The central atom has three atoms bonded to it and no lone pairs.
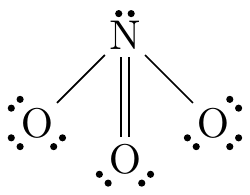
Trigonal pyramidal
The central atom has three atoms bonded to it and a lone pair.
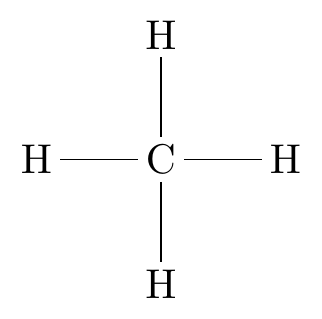
Tetrahedral
The central atom has four atoms bonded to it.
Bent
The central atom has two atoms bonded to it and at least one lone pair.
