Lewis dot diagrams
Lewis dot diagrams show the bonding between atoms in covalent molecular and ionic structures.
Covalent
Firstly consider a covalent structure. The method for drawing a dot diagram is as follows.
We will use the example of Carbon Dioxide, .
Step 1
Count the total number of valence electrons across all the atoms in the molecule. Take into account the charge. For example, if you were drawing a Lewis dot diagram for , you would subtract 1 from the number given here, since there is 1 less electron.

As is visible in the diagram above, the total number of valence electrons is .
Step 2
Count the number of electrons required to fill the outer shells of all of the atoms.
Using the above diagram, we can see that electrons are required.
Step 3
Calculate the number of bonds that will be in the final molecule.
Each bond requires two electrons, so we can calculate this using (number obtained in step 2 - number obtained in step 1) / 2. Using the numbers above: bonds will be required.
Step 4
Choose a molecule to go at the center of the structure; usually the atom with the highest valence or the least electronegative atom. In this case that atom is Carbon.
Step 5
Draw the bonds between the atoms (ignore valence electrons for now). Do this so that as many atoms as possible gain enough electrons to have a full outer shell. For example, Oxygen should have two outgoing bonds, since it needs two electrons for a full outer shell; Carbon should have four bonds.

Step 6
Add in the valence electrons to complete the octets of the outer atoms.
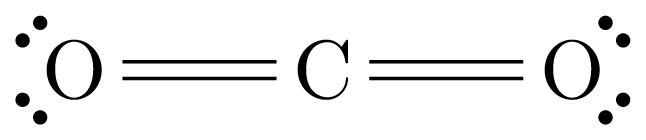
Step 7
Place any remaining electrons around the central atom. In this case this is unnecessary. Some of the bonds can be 'shifted' if the central atom does not have enough electrons. In this case the diagram is complete already.
Step 8
If the molecule originally had a charge, draw it in square brackets and add the charge.
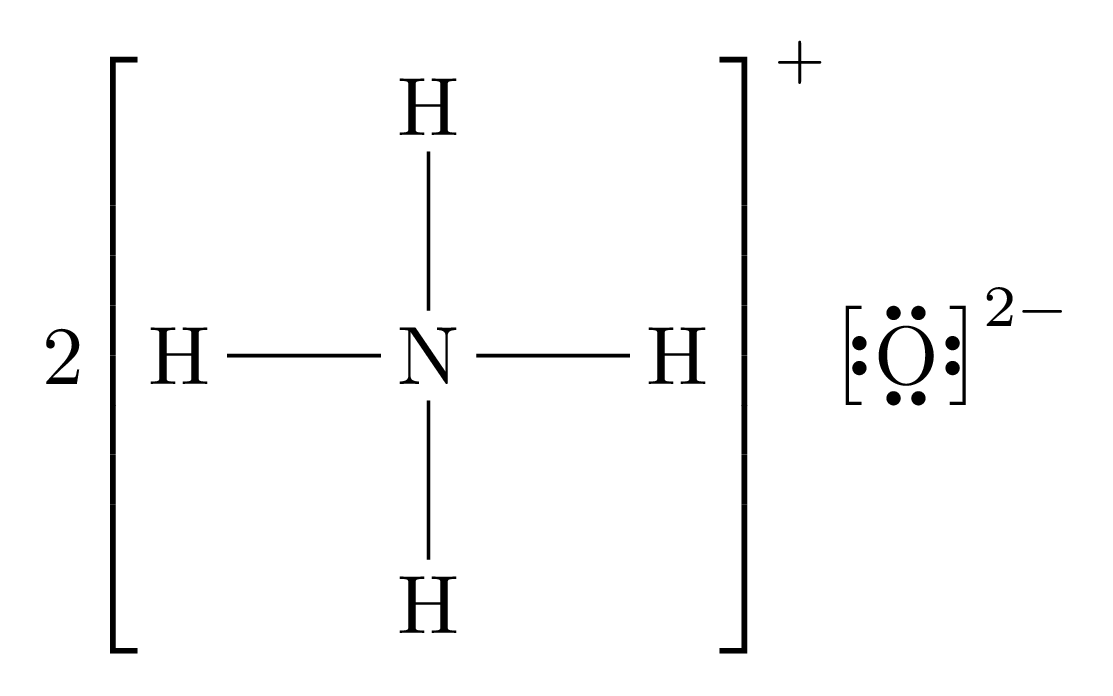
For example, the completed Lewis dot diagram for Ammonium () would look like this:

Ionic
We will use the example of Ammonium Oxide () for this demonstration.
Step 1
Break the ionic compound up into its positive and negative components, and create Lewis dot diagrams for each of those. Ensure that you write the charge for the molecules. If one of the molecules is a single atom, draw its charge. If it is a positive ion, draw no outer shell electrons; if it is a negative ion, draw a full outer shell.
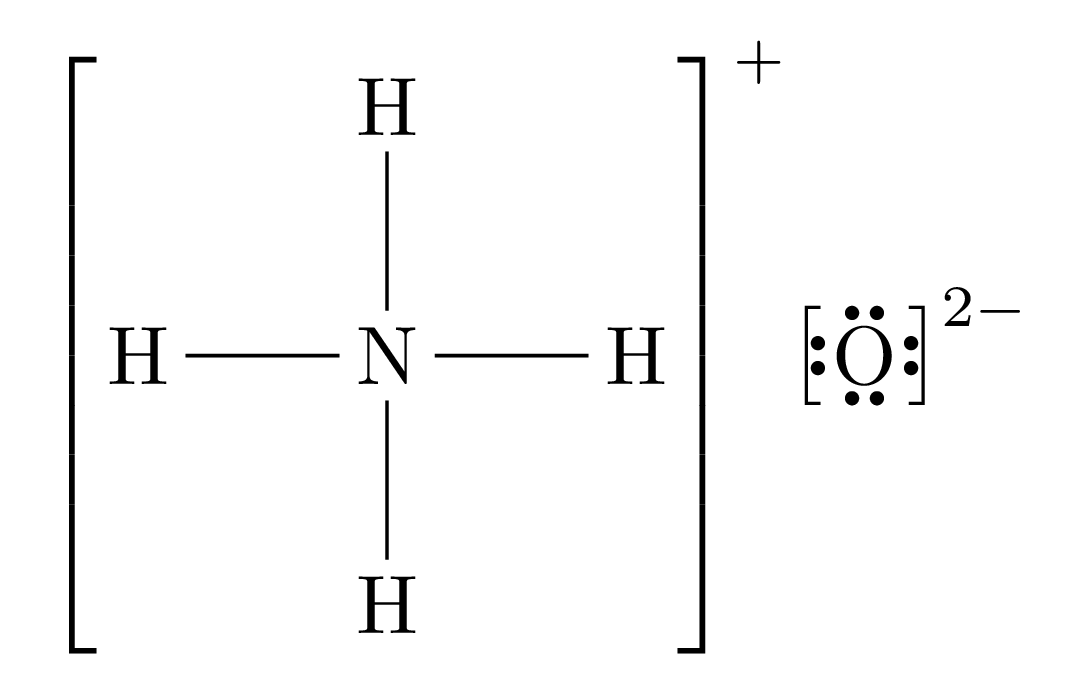
Step 2
Balance the diagram.